This million-dollar clinical study will utilize Type Zero’s inControl AP diabetes management platform (which we first told you about in June 2015) as the core analytic and control technology. Originally known as the DiAs (Diabetes Assistant system) and developed from a prototype licensed from the University of Virginia (UVA) in 2013, the startup changed the name last year to inControl.
Slated to kick off in the first half of 2016 and managed by Dr. Boris Kovatchev at the Center for Diabetes Technology at UVA, the study is called International Diabetes Closed Loop Trial (IDCL). It will include multiple sites — two in the United States and three internationally in France, Italy, and the Netherlands. It will test InControl with 240 people during a six-month at-home study, setting the stage with data for a final regulatory submission in 2017; even though the trial’s is set to last 2.5 years, the first six months of data gathered will likely be used to make an FDA filing.
The full system being studied uses insulin only and runs a control algorithm on an Android smartphone that communicates with a Roche or Tandem insulin pump as well as a Dexcom CGM. We’re told the system is ”pump agnostic” so they will test two or three other pump models in the mix as well. Study participants will ”treat-to-range,” meaning they will only be responsible for setting meal boluses, while the system will work to keep the patient’s glucose levels within a designated range by tweaking insulin basals automatically. Overnight, it will be programmed to keep BGs between 110-120 mg/dL (nice!).
This is the system that some may remember in its prototype phase as looking a bit like a stoplight. A new mobile smartphone app that communicates with the pump and CGM has replaced that original UI, with a design that is ”more commercially appealing.”
Announced during the week of ATTD, TypeZero is collaborating with Cellnovo Group that creates an insulin patch pump to use during this upcoming ICL trial.
While this study hasn’t yet begun, nor is info posted yet on ClinicalTrials.gov, you can learn more or sign up by contacting the research group directly at artificialpancreas@virginia.edu.
Bigfoot Biomedical
Finally, we’re able to get a view of the exciting system being developed by Bigfoot Biomedical, that startup launched in late 2014 that’s headed by former JDRF CEO Jeffrey Brewer, dubbed ”the father of the Artificial Pancreas.” Bigfoot, which now has 35 employees, was co-founded by four D-Dads: Brewer; Bryan Mazlish, the legendary “Bigfoot” himself, who invented a working AP prototype for his wife and son; former Medtronic chief engineer Lane Desborough, and former WellDoc CFO Jon Brilliant.
For the past year our D-Community has been abuzz with talk of Bigfoot, especially after the small startup acquired the former Asante Snap tech after that company went under last year — and then Bigfoot announced it was relocating from the East Coast to take over Asante’s abandoned Silicon Valley headquarters.
Bigfoot’s been busy this January presenting at key health and technology conferences, unveiling what it’s future tech will look like. The first 200 prototypes were rolling off the production line for clinical trial even as we talked with Jeffrey Brewer the other day by phone.
He tells us the system will have three main components:
The idea of a ”closed-loop” system for diabetes management that would automate insulin delivery once looked like a pipedream. But today, it’s a major focus of active development, with experts racing to achieve this “holy grail” faster than we’ve ever imagined.
2016 actually marks a decade since the launch of the JDRF Artificial Pancreas Project, which charted a roadmap for industry and academia. And it’s been incredible to see the explosion in do-it-yourself efforts on this front — core of the #WeAreNotWaiting grassroots initiative that has taken diabetes tech and healthcare by storm.
About two dozen Artificial Pancreas projects exist worldwide, but many of them are specialized research efforts that don’t capture headlines as much as the established insulin pump companies and hot startups: Bigfoot Biomedical on the West Coast (utilizing former Asante Snap insulin pump tech); TypeZero Technologies that spun out of Virginia academic research; and the iLET Bionic Pancreas based in Boston that uses dual hormones in its system.
Here’s a look at where these top projects currently stand:
TypeZero Technologies
With the start of the year came the big news that the National Institutes of Health (NIH) — you know, the U.S. government — is providing $12.7 million (!) in federal funding for closed-loop research involving Virignia-based TypeZero Technologies. Wow, that’s quite the investment!
- ”Bigfoot Brain” – containing the pre-filled insulin cartridge and tubing (this is the part based on former Asante Snap tech that Bigfoot acquired in mid-2015)
- Mobile App – acts as the controller and user interface
- Dexcom G5 – and eventually, future CGM generations
Jeffrey tells us they’re utilizing the disposable Snap insulin pump body, maintaining use of a pre-filled pen cartridge and the auto-priming function that Asante users liked so well.
”What goes away is the durable controller part that had the antiquated interface with the buttons and Atari screen,” he says. ”That goes into the museum with the Medtronic products.”
Instead, the ”Bigfoot Brain” will have a slick cover, with a microprocessor housed inside that contains all the smarts for controlling and calculating insulin dosing and other diabetes decision-making. A Bluetooth chip will allow it to talk to the smartphone, BG monitor, and Dexcom CGM.
”The device itself won’t have a screen or any buttons, as that will all be done on the phone that becomes the interface. That’s where you’ll enter meals, or keep track of how the system is doing, or where you’ll see announcements for glucose warnings, infusion set and CGM changes, calibrations, etc.,” he says.
OK, but what if you forget your phone? No worries, Jeffrey tells us, the system will keep working even without the phone nearby — it simply means you wouldn’t have access to your ”window” for a while. (Think of it like using the OmniPod tubeless pump and forgetting your PDM at home; the system keeps working with pre-programmed settings, but you just can’t see the data or give more insulin boluses if you don’t have the PDM with you).
The Bigfoot app will display your current CGM value and also a projected CGM reading for the next half hour.
During setup, it will ask the user: ”What’s your basal rate?” and ”How afraid are you of hypos?” Both are designed to set the parameters and tell the system how aggressive you want to be in dosing insulin. Text notifications are also being weaved in, we’re told.
Jeffrey says Bigfoot plans to use a monthly subscription model for this tech service.
”The subscription will cost less than everything costs today when you add up pump, CGM, and BGM plus supplies… considering that a $6,000 pump needs to be amortized over its four-year life,” he says. ”Bottom line is that paying by the month is better for consumers and insurance companies. You also have the benefit of getting the latest version of everything immediately, including software and hardware, rather than having to wait for authorization to get an upgrade.”
Bigfoot will start user research in the second quarter of 2016, in a selected Clinical Research Center (CRC) study that happens in-clinic and involves a lot of patient supervision. By the end of this year, Bigfoot plans an off-site study in which patients will stay in a hotel, but will have ready access to help with the system if needed.
If all goes according to plan, Bigfoot plans to start its pivotal trial (leading into FDA submission) in the first half of 2017 and it could be looking at a launch in 2018!
OMG…Only two years out — that’s freakin’ close!
iLET Bionic Pancreas
There are also new developments on the iLET Bionic Pancreas, led by the now famous D-Dad Dr. Ed Damiano and his team in Boston.
The very cool iLET system was unveiled at the Children With Diabetes Friends For Life conference last summer, and we took a deeper look at this closed-loop tech in October. Now, we’re told that the next-gen iLET will be ready by June!
We talked to Ed by phone this past week, just as he was wrapping up a very small clinical study at Stanford testing the device’s insulin-only capabilities. That new data is being presented at the big ATTD (Advanced Technologies & Treatments for Diabetes) conference taking place in Italy this week by also-famous Stanford endocrinologist Dr. Bruce Buckingham.
Ed tells us that since July, his team has been hard at work developing the iLET 3 (to replace the iLET 2 shown at FFL). The iLET 3 version is smaller and more compact, he says.
“That device you held at FFL was much larger with a 4.1-inch display, and as a result it was power-hungry and burned through AAA batteries,” Ed says. “This newest one will be thinner and lower power, closer to what we plan to eventually launch, and it will have a 3.2-inch screen with higher resolution, black and white LED display.
Of course, it will have the Dexcom G5 integrated inside.
Team iLET is also working on a proprietary infusion set, which will have a single needle and dual tubing for both glucagon and insulin to be administered. Ed says they have an application pending now before the FDA, to have the infusion set tested in a human study. They should know by the end of February when that small, targeted study can begin. It will include 10 people wearing the infusion set and using the iLET for 8 hours around Boston.
The team is also finishing up a clinical “set-point” study by mid-year in which people can designate their own BG ranges using the iLET, and by year’s end, they’ll begin their first human home-based “bridging study” that’s being funded by NIH and will include both kids and adults at four clinical sites across the U.S. – Massachusetts General Hospital in Boston (where this ”Team Bionic” is based); Stanford University in Palo Alto, CA; Nemours in Jacksonville, FL; and the Barbara Davis Center in Southern California.
The insulin-only component that they’ve just finished studying is also an important clinical focus, because it provides the core functionality to keep patients safe, Ed says.
“You could have an occlusion in the glucagon path, or if you’re out and don’t have access to it right away… iLET would need to fall back to an insulin delivery mode without glucagon. In these studies, it’s looking for glucagon every 5 minutes but not seeing any, so this is baked right into our algorithm.”
In fact, FDA specifically requested to see study results on the insulin-only delivery aspect, compared to ”regular treatment” with this dual-chamber system, so that will be built into the big pivotal trial planned for 2017. Instead of two study arms with people using iLET and those not, they’ll also have a third arm including patients using iLET’s insulin-only delivery. That pivotal trial will tentatively include 640 people — 40 people at each clinical site, with 20 on the dual hormone protocol, 10 on insulin-only, and 10 using traditional non-iLET treatment.
Ed says the insulin-only component of iLET actually opens up a channel for quicker commercialization, as they could release an insulin-only system first and then later launch the dual-hormone version once it gets FDA approval.
Nice progressive thinking there!
Established D-Industry
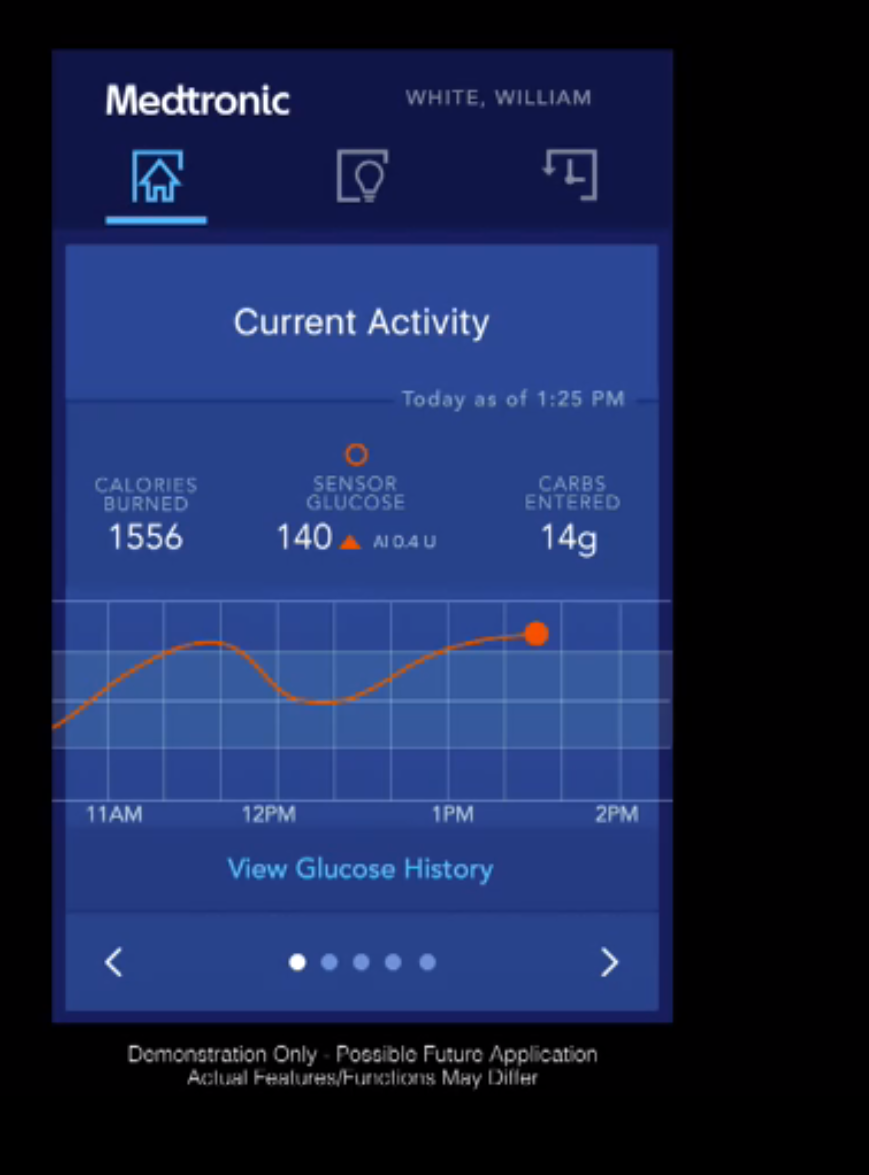 Medtronic: We’ve reported on the remarkable progress MedT is making on the closed-loop front, as recently as our coverage from the big Consumer Electronics Show at the start of the year, where the IBM Watson-powered mobile app with glucose predictive capabilities was on display. This super computer apparently has potential to predict hypos three hours before they happen — and MedT expects to launch the app with that capability this summer. Meanwhile, the company tells us they plan for an FDA filing soon of their next-generation Minimed 640G system that can predict hypos 30 minutes in advance.
Medtronic: We’ve reported on the remarkable progress MedT is making on the closed-loop front, as recently as our coverage from the big Consumer Electronics Show at the start of the year, where the IBM Watson-powered mobile app with glucose predictive capabilities was on display. This super computer apparently has potential to predict hypos three hours before they happen — and MedT expects to launch the app with that capability this summer. Meanwhile, the company tells us they plan for an FDA filing soon of their next-generation Minimed 640G system that can predict hypos 30 minutes in advance.
We’re told the smarts in that system are key to the Minimed 670G hybrid closed loop tech, anticipated by mid-2017. That will include the Israel-based DreaMed tech acquired last year, and the GlucoSitter software that uses a proprietary MD Logic Artificial Pancreas algorithm, now being built into the MedT closed-loop system.
We haven’t seen any images of the 670G floating around yet, but would have to guess that it will resemble the Minimed 640G predictive system that’s already launched outside the U.S. and is expected to hit the States within the next year.
Animas: The company is presumably still working on their Hypo-Hyper Minimizer (HHM), although we haven’t heard much — nor seen any images — since they last talked openly about this predictive system three years ago.
We reached out to Bridget Kimmel, Animas’ senior manager of communications and public affairs, and were told:
“Animas’ partnership with JDRF has enabled development of best-in-class predictive algorithms for closed-loop insulin delivery. Three feasibility clinical studies have been completed and results have been published. The Animas team is driving this technology aggressively to pivotal clinical studies and remain committed to continued innovation that addresses the needs of all our patients.”
While a new HHM research article was just published in the January 2016 issue of the Journal of Diabetes Science and Technology, and Animas is also scheduled for an oral presentation at ATTD in Italy this week, there’s very little new information being circulated.
The study published involved 12 adults with type 1 diabetes using a closed-loop system with HHM in a clinical research center for approximately 24 hours, and the Minimizer definitely succeeded in ”taking preemptive action to prevent hypoglycemia based on predicted changes in CGM glucose levels.” Good!
Now show us a little more of what’s in the works, Animas, if you want to stay in the closed-loop race.
Tandem: Maker of the touchscreen t:slim insulin pump is moving in the closed-loop direction with their recently released t:slim G4 CGM integrated product. As far as pipeline, company execs stay pretty mum, other than comments in investor earnings calls that hinted they’d either pursue a combo system with low-glucose suspend feature or a full closed loop basal algorithm.
In an earnings call last October, Tandem indicated it had planned to submit the ”first AP generation” for investigational purposes to the FDA in late 2015. We asked late last week for an update, but Tandem declined to answer based on regulatory rules that prohibit them from commenting until their next earnings call, sheduled for Feb. 24.
Insulet: The OmniPod tubeless insulin pump company has been equally mum on detail about what it has in the works, but continues to say how closed loop tech is ”on the radar” with a yet-to-be-named development partner. We hear that Insulet is once again working towards closer integration with the Dexcom CGM, presumably aiming to bringing it all together with a smartphone app for system control and data sharing. At least, that’s what we have to think they’re doing…
Others: We remain curious whether Roche Diagnostics or Abbott will move into this closed loop universe, but so far nothing we’ve heard indicates they’d be serious contenders.
That said, during a recent earnings call, Roche did finally make mention of its interest in CGM tech, and possibly something that could lead to a closed loop system; they presented a slide about launching the new Accu-Chek Insight CGM in Europe during this year, but of course left the details to our imagination. We can only assume (hope?!) the company’s got a plan to weave everything together with its Accu-Chek Insight pump system and eventually bring that to the States…
So as always, TBD.
Artificial Pancreas Progress: Key Takeaways
As mentioned, many more AP projects are happening across the globe, and we are rooting for every single one of them.
Agreeing with that is Dr. Aaron Kowalski, a longtime type 1 who is Chief Mission Officer and closed loop champion at the JDRF.
 ”We are obviously excited about AP-everything,” Aaron says. “The way the JDRF thinks about this is, everyone agrees that no matter which system you’re talking about, you can pretty much walk away and diabetes is handled for you. It is the definition of holy grail in that while it’s not a cure, it’s as close as tech can bring us without getting there!”
”We are obviously excited about AP-everything,” Aaron says. “The way the JDRF thinks about this is, everyone agrees that no matter which system you’re talking about, you can pretty much walk away and diabetes is handled for you. It is the definition of holy grail in that while it’s not a cure, it’s as close as tech can bring us without getting there!”
Aaron concurs that no commercial AP system is going to become available within the next year, but he’s excited to see them moving beyond academic research and heading into priority company development pipelines, regulatory trials and ever closer to FDA approval.
We are 100% on that same page, and can’t wait to see more updates – including what comes out of the big @ATTD2016 conference in Italy this week, where word is that ”closed loop” will be at the forefront.
See more on www.healthline.com
Nyhetsinfo
www red DiabetologNytt

