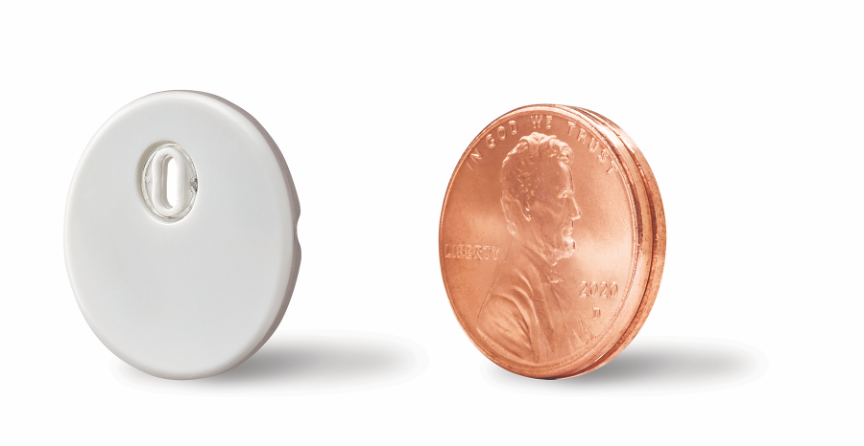Abbott announces CE-Mark for FreeStyle Libre 3,
smaller, thinner, and with real-time glucose and trend arrow information
September 28, 2020
Nedan info från amerikansk medicinsk-teknisk nyhetsw-ww,
läs hela info nedtill – och utdrag aktuell info finns också nedtill
Nyhetsinfo
www red DiabetologNytt
Executive Highlights
- Abbott just announced moments ago CE-Marking for its next generation FreeStyle Libre 3 device. FreeStyle Libre 3 is the first FreeStyle Libre product to offer full, real-time capability, removing the “scanning” requirement that has long been associated with Abbott’s CGM systems. FreeStyle Libre 3 is also 70% (!) smaller than the first two FreeStyle Libre systems (~two stacked US pennies vs. ~two stacked US quarters). Impressively, the device also keeps the same pricing, two-week wear, factory calibration, and pediatric and pregnancy indications in Europe as previous systems.
- Abbott plans to launch FreeStyle Libre 3 in the “coming months.” Abbott’s shared little information on FreeStyle Libre 2 uptake in Europe and it’s unclear how quickly Abbott plans to roll out the device though presumably it would be before year-end. With FreeStyle Libre 2, Abbott launched first in Germany and Norway, with broader rollout coming a few months later; Abbott didn’t outline any specific countries for initial launch of FreeStyle Libre 3 but we imagine Germany would again be first. See a 41-second marketing video here for a little more on the system. Freestyle Libre and Freestyle Libre 2 will both continue to be available OUS “for the duration,” as we understand it – there are fewer “bells and whistles” associated with Freestyle Libre and we assume it will be able to be offered in large volume slightly more cheaply than Freestyle Libre 3 though we have no actual information on this.
- The acceleration has been very rapid in CGM for so many of the top companies in the field; while the addition of real-time data in FreeStyle Libre 3 ramps up pressure on Dexcom/Verily to deliver on G7, we remind readers that G6 can still barely be kept in stock. By feature set, Abbott and Dexcom have been leading Medtronic, and with FreeStyle Libre 3 adding real-time glucose and trend arrow data, Abbott has a very strong case for having the most compelling CGM offering currently available particularly given cost. Assuming G7 delivers on its expected features, the device would certainly at least close the gap with FreeStyle Libre 3; while outside the US, FreeStyle Libre 3 will be available in the “coming months,” Dexcom’s G7 remains slated for a launch in 2021 – we won’t hold our collective breath for either soon, and again, we remind readers that demand is robust for both. Notably, Abbott’s FreeStyle Libre 3 can be used with closed loop systems in the EU, as we understand it, unlike in the US, where Dexcom has a clear advantage among the most intensively managed patients, just as Abbott as a clear advantage with the most cost-conscious patients and with the systems valuing lower prices.
- Notably, Abbott’s accuracy data from ATTD was published earlier this month – see “Accuracy of a 14-Day Factory-Calibrated Continuous Glucose Monitoring System With Advanced Algorithm in Pediatric and Adult Population With Diabetes”
- https://journals.sagepub.com/doi/abs/10.1177/1932296820958754
- in the Journal of Diabetes Science and Technology, September, 2020, by authors including Dr. Mark Christiansen of Walnut Creek (highly regarded local clinical trialist in the Bay Area), Dr. Greg Forlenza of IDC – Abbott researchers Hanqing Liu and Shridhara Alva were the last and first authors, respectively. This data was first referenced in Closer Look in February, 2020.
- See more, including pictures, a feature comparison (between FreeStyle Libre 3, FreeStyle Libre 2, Dexcom G6, Dexcom G7, Medtronic Guardian Sensor 3, and Senseonics Eversense XL) table, and Close Concerns’ questions below.
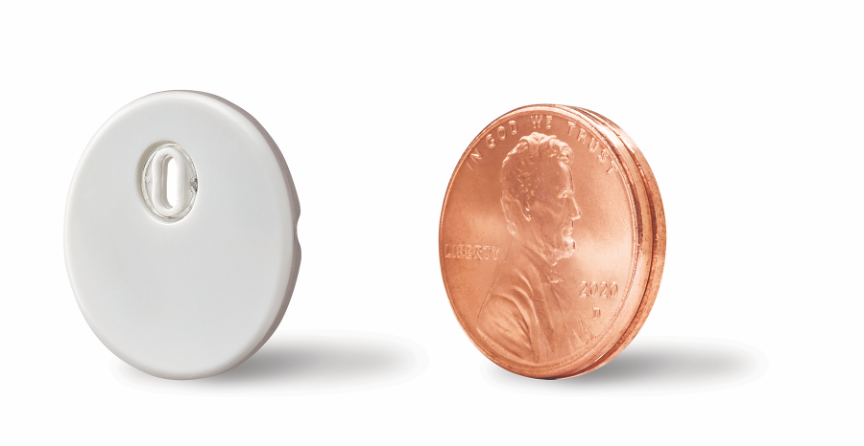
Table of Contents [hide]
-
- 1. “Smallest and thinnest” available sensor sends real-time minute-by-minute readings directly to FreeStyle Libre 3 smartphone app; “unsurpassed” 14-day accuracy
- 2. FreeStyle Libre 3 to launch in “coming months”; list pricing remains same as FreeStyle Libre 2 and FreeStyle Libre, a big deal given the improvements
- 3. Competitive Implications: FreeStyle Libre 3 strengthens Abbott’s OUS position
- 4. Feature Comparisons between FreeStyle Libre 3, FreeStyle Libre 2, Dexcom G6, G7, Medtronic Guardian Sensor 3, and Senseonics Eversense XL
- 5. Pictures of FreeStyle Libre 3
- 6. Close Concerns’ Questions
1. “Smallest and thinnest” available sensor sends real-time minute-by-minute readings directly to FreeStyle Libre 3 smartphone app; “unsurpassed” 14-day accuracy
FreeStyle Libre 3 preserves the same optional alarms, 14-day wear, and high accuracy (likely meeting iCGM criteria), while also adding real-time readings sent directly to a mobile app in a 70% smaller (and more discreet) sensor. The third-generation CGM sensor sends real-time, minute-by-minute readings directly to mobile devices via Bluetooth. This is a huge step forward for Abbott: although FreeStyle Libre 2 also has Bluetooth connectivity, which enables optional alarms, the system still required users to scan their sensor at least every eight hours to view their glucose values. By moving from scanning to Bluetooth with FreeStyle Libre 3, Abbott removes what some saw as a burden of scanning, which for many users, was a significant differentiator between FreeStyle Libre and other real-time offerings although some preferred it – presumably they’d continue to get FreeStyle Libre 2. In FreeStyle Libre 3, real-time data is directly sent to the FreeStyle Libre 3 mobile app, which is new with the updated system (since scanning is no longer required) and is available for both iOS and Android devices. Like LibreLink, which was used for FreeStyle Libre and FreeStyle Libre 2, the FreeStyle Libre 3 app enables users to view their real-time data (for the first time without scanning), glucose history, and trend arrows, all features which have been highly popular with earlier FreeStyle Libre models and apps.
- The FreeStyle Libre 3 sensor will also be 70% (!) smaller than previous models – “the smallest and thinnest” CGM sensor yet. Per the press release, FreeStyle Libre 3 is “the size of two stacked U.S. pennies” whereas FreeStyle Libre “1” and 2 are the size of “a stack of two U.S. quarters” (see pictures below). We’re looking forward to trying it; generally, a smaller CGM has obvious benefits for users: it is more discreet and fits better under clothes, making its use less burdensome and inconvenient for users, although the FreeStyle Libre 2 was already very small and didn’t have any sort of opening in it; we don’t think that will be a big deal but it is noticeable.
- The smaller size makes the sensor more sustainable, with a 41% reduction in plastic and a 43% reduction in carbon paper – the negative environmental impact of various diabetes products has become increasingly important to multiple users and it’s nice to see the progress here as well as the acknowledgement of these interests.
- FreeStyle Libre 3 retains many of the popular features that came with previous models. The system offers very good accuracy (9.2% MARD – such a far cry of BGM systems of old), non-adjunctive labeling, optional app-based alarms, and 14-day wear. With its real-time CGM design, this makes FreeStyle Libre 3 the longest-lasting real-time CGM available on the market. The product is indicated for upper arm wear only – ideally, Abbott will now push for abdomen wear approval given that scanning is no longer part of the process and abdominal wear would make the product even more discreet to users who value that. FreeStyle Libre 3 also retains the 1-hour warmup and factory calibration that came with FreeStyle Libre 2. It would be interesting to compare day 1 accuracy with other systems.
- Like FreeStyle Libre 2, FreeStyle Libre 3 will have a pediatric indication for ages 4+.
- As well, FreeStyle Libre 3 will also be approved for use in gestational diabetes and in pregnant women; from our view, every pregnant woman at any risk of gestational diabetes should wear this early in pregnancy (not waiting until week 26 as often happens with the test) and CGM should be standard of care in all type 1 pregnancies with an emphasis on practice guidelines and education and awareness around the technology so that it’s translated well.
- A reader won’t be necessary, which is great news for Abbott on the cost side; for patients that would rather use a reader, they can do so with the FreeStyle Libre 2. All FreeStyle Libre 3 users will use the app to access their data – this has become more common with CGM systems, as neither Medtronic’s standalone Guardian Connect, nor Senseonics’ Eversense XL have readers available. Given the popularity and ease of using as smartphone, many people would opt to use their smartphones as readers anyways; however, the move is a deviation from previous FreeStyle Libre models and from Dexcom G6, all of which had app and reader options for accessing data. We’ve heard the applicator is also improved (from a high base) and is now “all in one” – this is a positive application of the FreeStyle Libre and FreeStyle Libre 2 are easy, but not completely intuitive the first time they are used.
2. FreeStyle Libre 3 to launch in “coming months”; list pricing remains same as FreeStyle Libre 2 and FreeStyle Libre, a big deal given the improvements
Abbott didn’t provide many specifics on the hardware changes between FreeStyle Libre 2 and 3. We’d imagine the battery and/or transmitter on the FreeStyle Libre 3 might be more expensive than that of FreeStyle Libre 2 – there could also be added cost from miniaturizing the device. On the other hand, Abbott notes in its press announcement that the smaller size of FreeStyle Libre 3 reduces the total volume by 70%, plastic use by 41%, and carton paper use by 43%. Ultimately, it’s unclear how FreeStyle Libre 3 margins compare to FreeStyle Libre 2, but we salute Abbott’s ongoing commitment to global access with its same list pricing for all three offerings. Of course, what health systems are paying can be different from list price as we’ve seen in the UK where volume purchases by one system have enabled lower pricing.
- Abbott plans to launch FreeStyle Libre 3 in the “coming months.” Abbott’s shared little information on FreeStyle Libre 2 uptake in Europe and it’s unclear how quickly Abbott plans to roll out the device. With FreeStyle Libre 2, Abbott launched first in Germany and Norway, with broader rollout coming a few months later; Abbott didn’t outline any specific countries for initial launch of FreeStyle Libre 3. Abbott has made significant investments
- https://www.closeconcerns.com/knowledgebase/r/c92f8321#Record_433_million_in_Worldwide_FreeStyle_Libre_sales_up_64_YOY
- in manufacturing for FreeStyle Libre and of course, manufacturing for FreeStyle Libre 3 would have been part of those investments. Still, CGM as a whole is growing quickly on an ever-increasing base and we’d expect demand for FreeStyle Libre 3 to be quite high. Global penetration of CGM among all people with diabetes is still less than 5% though we imagine it must be approaching 50% of all with type 1. We continue to prioritize CGM for anyone on insulin or SUs or at minimum, anything that could prevent people with diabetes from landing in the hospital due to severe hypo.
- We wonder if payers will view FreeStyle Libre 3 as different from FreeStyle Libre 2 – presumably since it’s the same price, reimbursement would stay the same even in the worst case. Still, Dexcom has long positioned its G5/G6 as a “premium” product relative to FreeStyle Libre, and now with FreeStyle Libre 3 becoming “real-time” CGM in reality as well as perception, it will be interesting to see how payers view the systems. Each market in the EU is different, so we may see different approaches. Dexcom has also made it clear for ages that it is working on lower pricing also and it has great trust from intensively managed patients in particular who have long used it to power automated insulin delivery, which has enabled far better results even for patients that have traditionally had good management.
3. Competitive Implications: FreeStyle Libre 3 strengthens Abbott’s OUS position
With the no-Bluetooth, scan-based FreeStyle Libre “1,” the addition of alarms
https://www.closeconcerns.com/knowledgebase/r/c98c215a#Competitive_Implications_and_Positioning
in FreeStyle Libre 2, and now real-time data in FreeStyle Libre 3, Abbott has taken a step-wise to delivering its “always-on” CGM option. In our view, the FreeStyle Libre and the FreeStyle 2 had “real-time” sensing but some patients prefer to “scan” to get the number (which is continuously updated) rather than “hear about it” by alarm – the Libre 3 will just have it “always on” and always visible, rather than “on demand.” In hindsight, this seems like a very smart move, as the step-wise approach has allowed Abbott to offer FreeStyle Libre at lower cost. Given the public health implications, we’d rather everyone have either Freestyle Libre 2 or Freestyle Libre 3 and that they’d set the alarms, since we imagine time in range is easier to reach with alarms. Still, some want a more discreet experience and we expect both systems to continue to be popular – we’d expect Freestyle Libre, the first system, to be less popular over time. Lower cost has helped Abbott drive broad access and reimbursement for CGM technology and now, six years after CE-Marking for the original FreeStyle Libre system, Abbott is poised to bring its real-time CGM as well as to continue to bring its Freestyle Libre and Freestyle Libre 2 to those that want it to the existing 2+ million users and beyond. We also note – they all will have the same list price, and we imagine Abbott will be able to be more competitive with large healthcare systems who commit to a large volume.
- Importantly, Abbott will continue to make FreeStyle Libre 3 available at the “same affordable price as previous versions.” This matches with Abbott’s focus on pricing for “mass adoption,” and makes FreeStyle Libre 3 the CGM with the strongest set of features (longest wear, smallest form factor, fully disposable, no calibrations) also the highest value option – see our full feature comparison table below. Note that all prices are list prices and we do not know what is being offered to specific health systems except when they make it public, like NHS in England.
- Some believe the addition of real-time data in FreeStyle Libre 3 ramps up pressure on Dexcom/Verily to deliver on G7 – from our view, there’s little pressure since Dexcom sells as much G6 as it can manufacture, from our view. But, the clamoring for lower priced CGM certainly continues to get louder, and now Abbott will technically have a way to offer at least its Freestyle Libre (first) system at a lower price. By feature set, Abbott and Dexcom have been leading Medtronic, and with FreeStyle Libre 3 adding real-time glucose and trend arrow data, Abbott has a very strong case for having among the most compelling CGM offering currently available regardless of cost – Dexcom still rates very high for those who can pay for it, for multiple reasons, particularly its very highly rated Clarity app. That said – this is a big win for Abbott and really moves things forward. It reminds us of back in the day with CGM – this is akin to moving from 60 seconds to 30 seconds and adding a backlight. With FreeStyle Libre 3, Abbott now has the longest-wear, smallest size, only fully disposable, no calibration, real-time CGM. Though we haven’t seen accuracy data for FreeStyle Libre 3, the accuracy gap between G6 and FreeStyle Libre closed considerably with FreeStyle Libre 2. Assuming G7 delivers on its expected features, the device would close the gap with FreeStyle Libre 3; however, at least outside the US, FreeStyle Libre 3 will be available in the “coming months,” while Dexcom’s G7 remains slated for a launch in 2021. In other words, outside the US, Abbott will likely have at least a half-year head-start on building its already significant OUS market share.
- In the US, Abbott has not shared any potential timelines for FreeStyle Libre 3; however, the company has historically faced longer regulatory reviews in the US. For context, 14-day FreeStyle Libre was approved in the US nearly four years after its European approval (July 2018 and September 2014) and FreeStyle Libre 2 was approved in Europe ~1.5-years before it was cleared in the US (October 2018 and June 2020). Given Dexcom’s timing for G7 launch (sometime in 2021), it will be interesting to see when G7 enters the US market relative to FreeStyle Libre 3. We have no confirmation whether discussions with FDA have begun in earnest on FreeStyle Libre 3 – the company may want to get experience in the EU, though this is speculation
- There are no warnings or limitations against use of FreeStyle Libre 2 with automated insulin delivery systems in the European labeling. As Abbott has confirmed (and Insulet at EASD 2020), Abbott plans to be active in AID, though it’s hardly the only focus at present. From a technical standpoint, FreeStyle Libre 2 is capable of driving an AID system, as the device sends a signal via Bluetooth to a reader/smartphone continuously. While the FreeStyle Libre 2 device includes a “warning/limitation” against use with AID systems when cleared by the FDA, we imagine this may be as much a timing issue with FDA than a limitation of the system (a leader is still being sought to replace Dr. Alain Silk, CDRH is presumably very busy, former head Dr. Courtney Lias has been promoted, Dr. Stacey Beck now works at Dexcom, etc.). The “warning/limitation” on FreeStyle Libre 2 from the FDA is due to interference of high doses of ascorbic acid on FreeStyle Libre 2’s readings. Based on publicly disclosed timings, we’d imagine Abbott’s AID partners (Insulet, Tandem, Bigfoot) will likely be working on integrating their devices with FreeStyle Libre 3.
- Abbott will continue offering FreeStyle Libre 2 to users who prefer the “scanning” experience. At the same price and with a smaller form factor, we’d imagine that many will opt for FreeStyle Libre 3, but some will keep the “scanning” in FreeStyle Libre 2 – some may actually also prefer the flat and totally plain sensor as opposed to the smaller one though from the picture below, there is virtually no difference. Abbott has shared little information on uptake of its FreeStyle Libre 2 (instead sharing information about the entire “FreeStyle Libre franchise” user base), so we’re hopeful we’ll hear more about FreeStyle Libre 3 uptake as it launches.
- See more from Abbott on its blog this morning – “Freestyle Libre 3 sets new peak in diabetes care. The smallest, thinnest and most discreet continuous glucose monitor the world has ever seen.”
- https://www.abbott.com/corpnewsroom/product-and-innovation/freeStyle-libre-3-sets-new-peak-in-diabetes-care.html
4. Feature Comparisons between FreeStyle Libre 3, FreeStyle Libre 2, Dexcom G6, G7, Medtronic Guardian Sensor 3, and Senseonics Eversense XL
In this first table, we’ve summarized the key features of Abbott’s FreeStyle Libre 3 and FreeStyle Libre 2 and Dexcom’s G6 and G7 as a comparison given that these two companies hold ~82% of the growing CGM market (by revenue) as of 2Q20. For Dexcom G7, we’ve made certain assumptions about the product features based on publicly available data and statements as the device is not on the market. The yellow highlight denotes which system(s) has the advantage on a particular category; of course, this is our opinion, and some is subjective!
|
FreeStyle Libre 3 |
FreeStyle Libre 2 |
Dexcom G6 |
Dexcom G7 |
|
|
Fingerstick Calibration |
None – Factory Calibrated No optional user calibration in cases of sensor inaccuracy No sensor calibration code required |
None – Factory Calibrated No optional user calibration in cases of sensor inaccuracy No sensor calibration code required |
None – Factory Calibrated Each sensor has a unique calibration code – captured via photo |
None – Factory Calibrated |
|
Accuracy (MARD) |
9.2% |
9.3% |
9.0% |
– |
|
Labeling |
Non-adjunctive – Replaces fingersticks for treatment decisions |
Non-adjunctive – Replaces fingersticks for treatment decisions |
Non-adjunctive – Replaces fingersticks for treatment decisions |
Non-adjunctive – Replaces fingersticks for treatment decisions |
|
Population |
4+ years |
4+ years |
2+ Years |
– |
|
Warmup |
1 hour |
1 hour |
2 hours |
– |
|
Wear length |
14 days |
14 days |
10 days |
14-15 days (maybe 16?) |
|
Alarms |
Yes – real-time alarms on mobile app Threshold alerts for highs and lows |
Yes – real-time alarms on reader, must scan sensor to see glucose, eight-hour history stored on sensor patch Threshold alerts for highs and lows |
Yes – data sent continuously to two display devices – receiver and app Predictive alerts for lows |
Yes – data sent continuously to two display devices – receiver and app Predictive alerts for lows |
|
Data display |
FreeStyle Libre 3 mobile app (iOS and Android) for viewing real-time data |
FreeStyle Libre reader device and LibreLink mobile app (Android and iOS) |
Apps for Apple iOS and Android, plus smartwatch apps for viewing RT data G6 receiver |
Apps for Apple iOS and Android, plus smartwatch apps for viewing RT data G7 receiver? |
|
Transmitter design and on-body form factor |
~2 stacked U.S. pennies Fully disposable and integrated with sensor patch |
~2 stacked quarters Fully disposable and integrated with sensor patch |
Small eraser-sized Three-month use transmitter separate from sensor |
Fully disposable wearable (integrated sensor/ transmitter), slimmer “nickel-sized” |
|
Insertion and Approved Location |
Single-press inserter device with each sensor Upper arm only |
Single-press inserter device with each sensor Upper arm only |
Single-push-button applicator with each sensor Abdomen (adults) Abdomen and upper buttocks (children) |
Single-push-button applicator with each sensor |
|
Pricing without insurance (one-month supply) |
$109 |
$109 |
$350 |
– |
In this second table, we’ve compared the key features of the newest standalone CGMs approved in Europe from each of the major CGM companies: Abbott’s FreeStyle Libre 3, Dexcom G6, Medtronic Guardian Sensor 3, and Senseonics’ Eversense XL. The yellow highlight denotes which system(s) has the advantage on a particular category; of course, this is our opinion, and some is subjective!
|
FreeStyle Libre 3 |
Dexcom G6 |
Medtronic Guardian 3 |
Senseonics Eversense XL |
|
|
Fingerstick Calibration |
None – Factory Calibrated No optional user calibration in cases of sensor inaccuracy No sensor calibration code required |
None – Factory Calibrated Each sensor has a unique calibration code – captured via photo |
2/day |
2/day |
|
Accuracy (MARD) |
9.2% |
9.0% |
~9% (upper arm) |
8.9% |
|
Labeling |
Non-adjunctive – Replaces fingersticks for treatment decisions |
Non-adjunctive – Replaces fingersticks for treatment decisions |
Adjunctive – requires fingerstick confirmation |
Non-adjunctive – Replaces fingersticks for treatment decisions |
|
Population |
4+ years |
2+ Years |
14+ years |
18+ years |
|
Warmup |
1 hour |
2 hours |
2 hours |
24 hours |
|
Wear length |
14 days |
10 days |
7 days |
180 days |
|
Alarms |
Yes – real-time alarms on mobile app Threshold alerts for highs and lows |
Yes – data sent continuously to two display devices – receiver and app Predictive alerts for lows |
Yes – data sent continuously to Apple iOS app Predictive alerts for highs and lows |
Yes – data sent to app and on-body transmitter for vibration alerts Threshold alerts for highs and lows |
|
Data display |
FreeStyle Libre 3 mobile app (iOS and Android) for viewing real-time data |
Apps for Apple iOS and Android, plus smartwatch apps for viewing RT data G6 receiver |
Apple iOS app only; Apple Watch app only mirrors phone notifications No receiver |
Apps for Apple iOS and Android |
|
Transmitter design and on-body form factor |
~2 stacked U.S. pennies Fully disposable and integrated with sensor patch |
Small eraser-sized Three-month use transmitter separate from sensor |
~2.5 stacked quarters (clamshell) Rechargeable transmitter separate from sensor Significant on-body tape |
~3 silver dollars Rechargeable transmitter separate from sensor |
|
Insertion and Approved Location |
Single-press inserter device with each sensor Upper arm only |
Single-push-button applicator with each sensor Abdomen (adults) Abdomen and upper buttocks (children) |
Inserter device, requires pulling needle out manually Abdomen & upper-arm |
Sensor implanted every 90 days Upper arm |
|
Pricing without insurance (one-month supply) |
$109 |
$350 |
$345 |
$99 per 90-day sensor through Eversense Bridge Program |
5. Pictures of FreeStyle Libre 3